Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Industry Statistics: Growth, Share, Value, and Trends By 2032
Competitive Analysis of Executive Summary Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market Size and Share
- The Asia-Pacific drug safety solutions and pharmacovigilance market size was valued at USD 1.42 billion in 2024 and is expected to reach USD 2.65 billion by 2032, at a CAGR of 8.10% during the forecast period.
Global Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market report comprises of data that can be quite essential when it comes to dominate the market or making a mark in the market as a new emergent. The statistics are represented in graphical format in this report for a clear understanding on facts and figures. The report provides insights which help to have a more precise understanding of the market landscape, issues that may impinge on the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market industry in the future, and how to position specific brands in the best way. Analysis and discussion of important industry trends, market size, and market share estimates are mentioned in the wide-ranging Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market business report.
Market research analysis and insights covered in the reliable Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market report are very considerate for the businesses to make better decisions, to develop better strategies about production, Market, sales and promotion of a particular product and thereby extending their reach towards the success. With the use of outstanding practice models and excellent method of research to generate this report that aids businesses to uncover the greatest opportunities to prosper in the market. While preparing the widespread Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market report, no stone is left unturned to consider public demands, competencies and the constant growth of the working industry, vibrant reporting, and high data protection services.
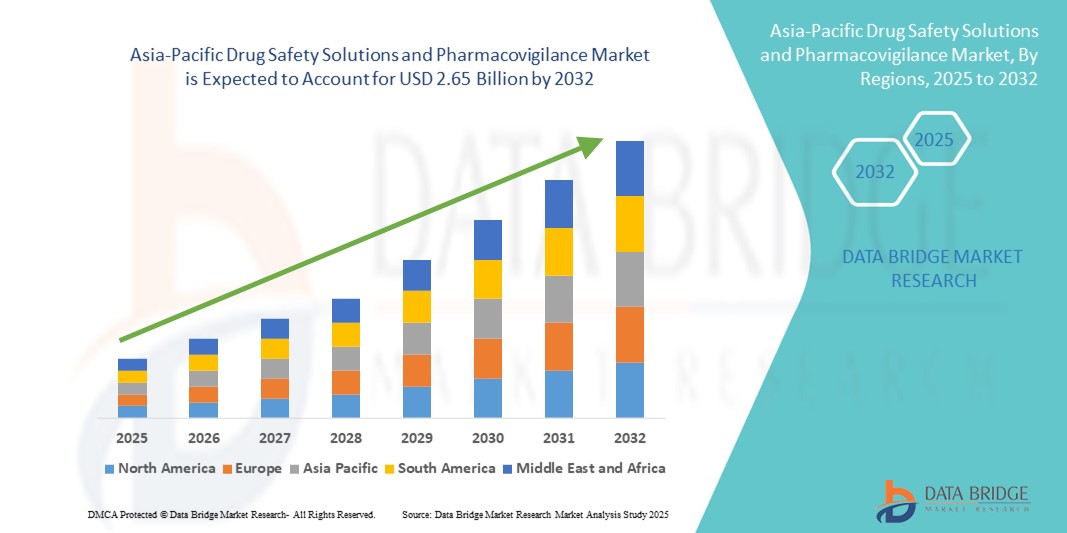
Get the edge in the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market—growth insights and strategies available in the full report:
https://www.databridgemarketresearch.com/reports/asia-pacific-drug-safety-solutions-and-pharmacovigilance-market
Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market Landscape Overview
Segments
- By Software Type: The market can be segmented based on software type into adverse event reporting software, drug safety audits software, issue tracking software, fully integrated software, and others. The adverse event reporting software segment is expected to hold a significant share as it helps in efficiently collecting and analyzing adverse event data to ensure drug safety and compliance with regulatory requirements.
- By Delivery Mode: The market can also be segmented by delivery mode into on-premise solutions and cloud-based solutions. Cloud-based solutions are gaining popularity due to their flexibility, scalability, and cost-effectiveness. They offer easy access to data from any location, making them highly preferred by pharmaceutical companies and CROs.
- By End-User: The end-user segments include pharmaceutical and biotechnology companies, contract research organizations (CROs), business process outsourcing (BPO) firms, and others. The pharmaceutical and biotechnology companies segment is anticipated to dominate the market due to the increasing focus on drug safety and pharmacovigilance to ensure patient safety and regulatory compliance.
Market Players
- IQVIA
- Oracle
- SAS Institute Inc.
- Ennov
- EXTEDO
- Online Business Applications, Inc.
- United BioSource Corp.
- Sparta Systems, Inc.
- Max Applications
- ArisGlobal
- Oracle Corporation
- Lingotek
- EXTEDO
- Ennov Solutions, Inc.
These key market players are actively involved in strategic collaborations, partnerships, and product innovations to enhance their market presence and expand their customer base in the Asia-Pacific region's drug safety solutions and pharmacovigilance market. The increasing focus on improving drug safety processes, stringent regulatory guidelines, and the rising demand for pharmacovigilance services are driving the growth of the market in the Asia-Pacific region. Market players are investing in advanced technologies, such as artificial intelligence and machine learning, to streamline drug safety processes and enhance operational efficiency.
The Asia-Pacific drug safety solutions and pharmacovigilance market is poised for substantial growth in the coming years, fueled by the increasing pharmaceutical outsourcing activities, expanding pharmaceutical industry, and rising focus on patient safety and regulatory compliance. The market players are focusing on expanding their product portfolios, strengthening their distribution networks, and investing in research and development activities to gain a competitive edge in the market. With the growing adoption of cloud-based solutions and advancements in software technologies, the Asia-Pacific region presents lucrative opportunities for market players to capitalize on the evolving needs of pharmaceutical companies and research organizations in ensuring drug safety and regulatory compliance.
The Asia-Pacific drug safety solutions and pharmacovigilance market is experiencing significant growth driven by several key factors. One of the primary drivers is the increasing focus on patient safety and regulatory compliance within the pharmaceutical industry. With stringent regulations in place to monitor and report adverse events related to drugs, there is a growing demand for sophisticated software solutions that can streamline the pharmacovigilance process. Market players are responding to this demand by investing in advanced technologies like artificial intelligence and machine learning to enhance efficiency and accuracy in adverse event reporting and analysis.
Furthermore, the expanding pharmaceutical industry in the Asia-Pacific region is contributing to the growth of the drug safety solutions market. As pharmaceutical companies continue to innovate and develop new drugs, there is a parallel need to ensure the safety and efficacy of these products. Drug safety software plays a crucial role in monitoring and managing adverse events throughout the lifecycle of a drug, from clinical trials to post-market surveillance. This reliance on technology to maintain high safety standards is propelling the demand for sophisticated pharmacovigilance solutions in the region.
Moreover, the trend towards outsourcing pharmacovigilance activities is also driving market growth in Asia-Pacific. Pharmaceutical companies are increasingly leveraging the expertise of contract research organizations (CROs) and business process outsourcing (BPO) firms to handle drug safety monitoring and reporting tasks. This shift towards outsourcing is creating opportunities for software providers to offer tailored solutions that meet the specific needs of these service providers, further boosting market expansion.
In terms of market competition, key players in the Asia-Pacific drug safety solutions and pharmacovigilance market are continuously striving to enhance their market presence through strategic partnerships, collaborations, and product innovations. These efforts are geared towards catering to the evolving requirements of pharmaceutical companies and research organizations in the region. By focusing on expanding their product portfolios, improving distribution networks, and investing in research and development, market players are positioning themselves to meet the growing demand for advanced drug safety solutions in the Asia-Pacific region.
Overall, the Asia-Pacific drug safety solutions and pharmacovigilance market present a promising landscape for market players looking to capitalize on the region's evolving pharmaceutical industry and regulatory environment. With a strong emphasis on patient safety, regulatory compliance, and technological advancements, the market is poised for substantial growth in the coming years. As market players continue to innovate and adapt to changing industry dynamics, they are well-positioned to drive further advancements in drug safety practices and contribute to the overall healthcare ecosystem in the region.The Asia-Pacific drug safety solutions and pharmacovigilance market is witnessing significant growth due to various factors driving the demand for sophisticated software solutions in the pharmaceutical industry. One key driver is the increasing emphasis on patient safety and regulatory compliance, leading to a higher demand for advanced technologies to streamline pharmacovigilance processes. Market players are investing in artificial intelligence and machine learning to enhance the efficiency and accuracy of adverse event reporting and analysis, reflecting the industry's shift towards more technologically-driven solutions.
Additionally, the expanding pharmaceutical industry in the Asia-Pacific region is contributing to the growth of the drug safety solutions market. With the continuous innovation and development of new drugs, there is a parallel need to ensure the safety and efficacy of these products. Drug safety software plays a critical role in monitoring adverse events throughout a drug's lifecycle, from clinical trials to post-market surveillance. This reliance on technology is boosting the demand for sophisticated pharmacovigilance solutions in the region to maintain high safety standards amidst industry advancements.
Moreover, the trend towards outsourcing pharmacovigilance activities is further propelling market growth in Asia-Pacific. Pharmaceutical companies are increasingly turning to CROs and BPO firms to handle drug safety monitoring and reporting tasks, creating opportunities for software providers to offer tailored solutions to meet the specific needs of these service providers. This shift towards outsourcing underscores the importance of flexible and scalable software solutions that can adapt to evolving industry requirements and support seamless collaboration between pharmaceutical companies and service providers in ensuring drug safety and regulatory compliance.
In terms of market competition, key players in the Asia-Pacific drug safety solutions and pharmacovigilance market are actively engaging in strategic partnerships, collaborations, and product innovations to enhance their market presence and better serve the evolving needs of pharmaceutical companies and research organizations in the region. By focusing on expanding their product portfolios, improving distribution networks, and investing in research and development activities, market players are positioning themselves to address the growing demand for advanced drug safety solutions in the dynamic Asia-Pacific market landscape.
Overall, the Asia-Pacific drug safety solutions and pharmacovigilance market offer promising opportunities for market players to capitalize on the region's evolving pharmaceutical industry and regulatory landscape. With a strong focus on patient safety, regulatory compliance, and technological advancements, the market is poised for substantial growth in the foreseeable future. As market players continue to innovate and adapt to changing industry dynamics, they are set to drive advancements in drug safety practices and contribute to the overall healthcare ecosystem in the Asia-Pacific region.
Study the company’s hold in the market
https://www.databridgemarketresearch.com/reports/asia-pacific-drug-safety-solutions-and-pharmacovigilance-market/companies
Custom Question Framework for Global Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market Reports
- What does the most up-to-date research indicate about Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market size?
- What is the expected yearly market acceleration?
- What are the major subdivisions discussed in the Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market report?
- Which businesses lead in competitive positioning?
- What innovations or launches have made an impact lately?
- What global and local markets are under review?
- Where is the fastest regional expansion occurring?
- Which country will likely be at the forefront by Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market size?
- Which region has the strongest historical Asia-Pacific Drug Safety Solutions and Pharmacovigilance Market Market base?
- What region or country shows the highest compound growth rate?
Browse More Reports:
Global Luxury Cosmetics Market
Global Polypropylene Market
Global Small-Scale Liquefied Natural Gas (LNG) Market
Global Vegan Dog Food Market
Global Women’s Footwear Market
Global Wood Based Panel Market
India Bakery Premixes Market
Europe Flat Glass Market
Europe Shower Enclosure and Cubicles Market
Global AI in Fashion Market
Global Automotive Wheel Rims Market
Global Banana Flour Market
Global Caffeinated Beverage Market
Global Cardiopulmonary Exercise Testing Market
Global Chemical Software Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"




